Feed mill hygiene is the first link in the global food safety chain. Contamination at this early stage can compromise animal health and, by extension, jeopardize the safety of the food that reaches the consumer's table.
In an industry where traceability and trust are essential, maintaining optimal hygienic conditions is much more than a legal obligation: it is a matter of competitiveness, reputation and sustainability.
European reports (EFSA/RASFF) show that feed is a critical link in the farm-to-table chain, with recurrent mycotoxin notifications, Salmonella and chemical pollutants; therefore, the EU requires HACCP and traceability specific to the sector.
A single incident can result in costs ranging from 100,000 to 2 million euros, considering product losses, production downtime, decontamination processes, regulatory sanctions and reputational damage (according to industry analysis and documented cases, such as the 1999 dioxin crisis).
Furthermore, feed contamination not only generates economic impact: its effect spreads throughout the food chain, affecting public health and causing health crises of international scope.
For a plant manager or production manager, hygiene must be understood not just as a regulatory requirement, but as a strategic investment in efficiency and safety.
Various industry studies, including those by GMP+ International and the IFIF (International Feed Industry Federation), estimate a return on investment (ROI) of between 3:1 and 7:1 for properly implemented industrial hygiene programs.
This return is derived from reduced waste, increased equipment life, improved productivity and prevention of potential plant shutdown incidents.
In a global context of increasingly stringent regulatory and market requirements, industrial hygiene has become an indicator of operational excellence. Companies that integrate it into their strategy not only comply with the law: they lead the market, gain in reliability and strengthen the confidence of customers, consumers and authorities.
International Regulatory Framework
Feed manufacturing hygiene regulations vary from region to region, but they share a common goal: to ensure safety throughout the food chain.
At the European level, the Regulation (EC) No. 183/2005 establishes the general hygiene and traceability requirements, while in Spain, Royal Decree 821/2008 adapts these requirements to the national context, defining the procedures for registration, authorization and control of manufacturing establishments.
In the United States, the Food Safety Modernization Act (FSMA) marks a similar preventive approach, based on the principle of “prevention rather than correction”, and incorporates specific controls for animal feed production.
At the international level, organizations such as the FAO and the IFIF (International Feed Industry Federation) promote the harmonization of standards through the Codex Alimentarius Code of Practice on Good Animal Feeding, which serves as a global reference for food safety management systems applicable to animal feed.
Together, these regulations form a framework of shared responsibility between producers, suppliers and authorities, which seeks to ensure that each stage of the process - from the reception of raw materials to the shipment of the final product - maintains the highest levels of hygiene and control.
Penalties for non-compliance in the EU can reach up to 600,000 €, in addition to the loss of international certifications such as GMP+, FAMI-QS or ISO 22000.
In highly regulated markets, these certifications are access requirements for large integrators and distributors.
Pathogens and Contaminants in the Feed Industry
The main biohazards in feed mills include bacteria such as Salmonella spp., Escherichia coli y Clostridium perfringens, in addition to mycotoxin-producing fungi.
These microorganisms can be introduced through contaminated raw materials, transport systems or residues accumulated in equipment and silos.
Salmonella is of particular concern because of its ability to survive more than 12 months in dry environments and form biofilms on metal or plastic surfaces, making it difficult to remove by conventional cleaning. Once present, it can easily spread through dust or pneumatic transport, contaminating different areas of the plant.
E. coli, Although it is usually an indicator of fecal contamination rather than a direct pathogen, it points to deficiencies in the hygiene of raw materials or in the cleaning process.
For its part, Clostridium perfringens, It is a spore-former, can resist heat treatment and proliferate in areas with accumulation of organic waste or poor ventilation.
As for fungi, species such as Aspergillus, Fusarium y Penicillium generate mycotoxins -aflatoxins, zearalenone, fumonisins- that pose a risk to both animal and human health.
FAO estimates that up to 25 % of agricultural raw materials may have detectable levels of mycotoxins, especially when grain is stored at high humidity or without adequate ventilation.
Chemical risks derive from detergent residues, heavy metals or industrial pollutants such as dioxins, while physical risks are associated with foreign bodies, dust or metal fragments generated during milling or transport.
| Type of pollutant | Examples | Favorable factors | Impact |
|---|---|---|---|
| Biologicals | Salmonella, E. coli, mycotoxins, mycotoxins | High humidity, prolonged storage, organic residues | Zoonotic risk and product loss |
| Chemicals | Cleaning residues, heavy metals, dioxins, dioxins, etc. | Incorrect dosing, cross-contamination, equipment wear and tear | Toxicity and regulatory sanctions |
| Physicists | Metal fragments, dust, stones | Equipment wear and tear, inadequate transportation | Mechanical damage and lot rejection |
Prevention at Every Stage of the Process
Hygiene control must cover all stages of production, from reception to final dispatch.
Reception
The reception of raw materials is one of the most vulnerable points in the process.
Each lot must undergo visual inspection, documentary verification and representative sampling prior to unloading.
Suppliers must be approved and have recent quality certificates and microbiological analyses, including the absence of Salmonella in 25 g and mycotoxin levels within EFSA limits.
It is recommended to use a vehicle access control and cleaning system, with automatic washing of wheels and hoppers. In addition, unloading surfaces should be cleaned between batches to avoid cross-contamination. All batch information (supplier, date, sample number and analysis results) should be recorded in a digital traceability system that allows tracing the origin of any incident.
A minimum sampling of 1 kg per 25 tons of raw material ensures statistical representativeness. Raw materials that do not meet the standards should be rejected or isolated for reanalysis.

Storage
Proper storage of ingredients and intermediate product is key to maintaining safety and nutritional stability. Environmental conditions should be kept within controlled ranges: relative humidity between 60-65 % and temperature below 25 °C; these conditions prevent the proliferation of fungi and the formation of condensation.
Temperature and humidity sensors with continuous recording should be installed and integrated into the plant's environmental management system. Warehouses should have positive pressure with respect to external areas and a minimum air renewal of 6 volumes/hour. Forced ventilation and correct inventory rotation according to the FIFO (First In, First Out) principle are essential to avoid lot aging.

Pest control should be outsourced to certified companies, with weekly inspections and documented records. Dry cleaning (vacuuming and sweeping) is prioritized over wet cleaning to avoid problems with residual moisture and microbial growth.
Mixing
The mixing room represents one of the most critical points in the prevention of cross-contamination, especially when both medicated and conventional feeds are produced on the same line.
Strict batch sequencing procedures should be applied, always starting with products of lower microbiological risk and ending with those of greater load or complexity.
Between each batch, the equipment must undergo mechanical cleaning and complete vacuuming of the residual material.
Visual inspection and surface sampling (swabbing) should verify the absence of visible or microbiological residues before the start of the next cycle.

The accuracy of mixing also influences safety: uneven distribution of additives or preservatives can create micro-zones with a high risk of contamination or degradation.
Therefore, homogeneity verification should be performed at least once a week by coefficient of variation analysis (CV < 5 %).
The entire operation must be documented and audited under standard operating procedures (SOP), with automatic records in the production control system.
Pelletizing and Thermal Hygienization
The pelleting process is a critical stage from the microbiological point of view.
Prior to pelletizing, the feed is passed through a sanitizer, where saturated steam is injected at controlled pressure.
This treatment raises the temperature of the product to between 75 °C and 85 °C for 90-120 seconds, ensuring the inactivation of most pathogenic microorganisms, particularly Salmonella spp. y E. coli. Unlike the traditional conditioner, whose objective is to improve the physical quality of the pellet, the sanitizer is specifically designed for microbial reduction, prolonging the thermal retention time of the product. Under these conditions, a greater than 99.9 % reduction of vegetative bacterial flora is achieved.
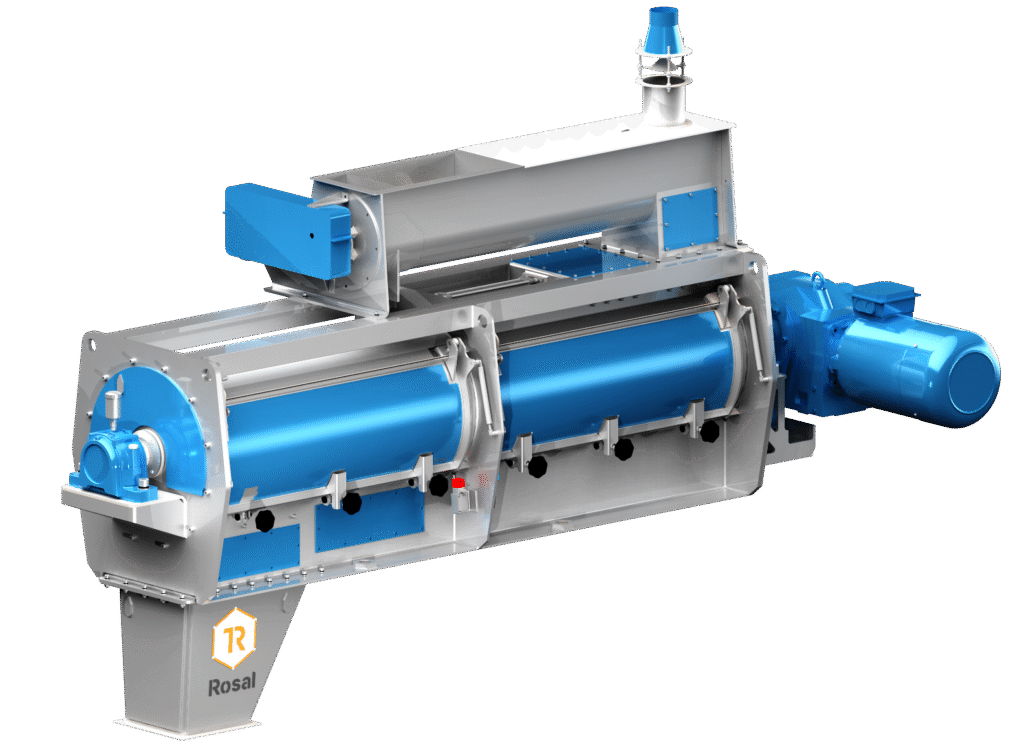
The sanitizer design must ensure homogeneous steam distribution and sufficient product retention, avoiding cold zones or accumulations that could compromise thermal efficiency.
Cooling, Screening and Dispatching
The cooling post pelleting should remove condensate and prevent recontamination of the product. The use of forced ventilation, clean drains and regular cleaning of screens guarantees the final quality of the feed.
During shipment, trucks, hoppers and loading equipment must present current cleaning and disinfection certificates, and operations must be recorded with date, lot and final destination.
| Stage | Main control | Critical parameters | Recommended frequency |
|---|---|---|---|
| Reception | Inspection and sampling | Certificates, Salmonella, mycotoxins, mycotoxins | Each lot |
| Storage | Environmental control | Humidity 60-65 %, ventilation | Weekly |
| Mixing | Prevention of cross-contamination | Cleaning between lots | Diary |
| Pelleting | Heat treatment with sanitizers | ≥ 75 °C | Per lot |
| Cooling / Screening | Condensate removal | Ventilation and screen cleaning | Each shift |
| Expedition | Vehicle verification | Cleaning certificates | Each charge |
Disinfectants and Application Parameters
The selection of chemicals must comply with Regulation (EU) 528/2012 on biocidal products and follow the recommendations of the European Chemicals Agency (ECHA).
Contact times and concentrations are critical variables for the effectiveness of the process. Insufficient concentration or reduced exposure time may allow microorganisms to survive, while too much may generate residues or damage surfaces.
Therefore, cleaning programs must specify precisely the doses, temperatures, times and methods of application, and their compliance must be periodically verified by microbiological analysis.
| Disinfectant | Concentration | Contact time | Application |
|---|---|---|---|
| Sodium hypochlorite | 100-200 ppm | 5-10 min | General surfaces |
| Quaternary ammoniums | 200-400 ppm | 10-15 min | Process equipment |
| Hydrogen peroxide | 0.5-3 % | 5-20 min | Critical areas |
| Isopropyl alcohol | 70 % | 30 s | Small tools |
The effectiveness of disinfection must be validated by systematic microbiological controls, confirming the reduction of the microbial load after each cleaning cycle.
The recommended reference values are:
-
Total mesophilic aerobic count: < 100 CFU/cm².
-
Enterobacteriaceae: absence in 25 cm².
-
Salmonella spp.: absence in 25 cm².
Compliance with these criteria demonstrates the effectiveness of the hygiene programs and constitutes documentary evidence for audits or certifications (GMP+, ISO 22000, FAMI-QS).
Hygiene Technology and Automation
Modern factories integrate automated technologies and digital control systems that reduce the risk of human intervention and increase the reproducibility and traceability of cleaning operations.
These tools allow for a data-driven approach, ensuring consistency of results and optimizing the use of resources.
- CIP (Clean in Place) systems are a clear example of this automation: they allow internal cleaning of equipment and piping without disassembly, reducing downtime by up to 50 % and water and chemical consumption by 20 to 40 %.
By operating in a programmed and electronically controlled manner, CIP systems ensure precise dosing of detergents, homogeneous temperatures and sufficient contact times, avoiding human error and increasing microbiological safety. - Disinfection by UV-C radiation (254 nm) has established itself as an effective complementary technology for the treatment of air and surfaces in critical areas.
With a minimum effective dose of 40 mJ/cm². and exposures of 15-30 seconds, it achieves a reduction of more than 99 % of vegetative microorganisms, without leaving chemical residues.
Its application is especially useful in ventilation ducts, storage chambers and areas where it is not possible to use water or liquid disinfectants. - IoT (Internet of Things) sensors provide continuous environmental monitoring, with accuracies of ±0.5 °C in temperature and ±2 % relative humidity, transmitting data in real time to monitoring systems.
These sensors can detect deviations before they become an operational problem, automate alarms and maintain auditable digital records.
In parallel, HEPA filters guarantee optimum air quality, with a 99.97 % efficiency for 0.3 µm particles or above, avoiding the introduction of dust or fungal spores in clean areas.
All these parameters are integrated into ERP or SCADA management and supervision systems, allowing centralized digital monitoring, remote control of cleaning cycles, and complete traceability of each operation.
Thanks to this digitization, plants can document protocol compliance in real time, generate automatic alerts and demonstrate the effectiveness of their measures to audits or international customers.
Organizational Culture and Training
Biosecurity in feed mills depends on both technology and human behavior. No automated system is a substitute for hygienic discipline and continuous training of personnel.
Each employee must receive a minimum of 40 hours of initial training in industrial hygiene and 16 hours of annual refresher training, covering cleaning practices, cross-contamination control and the safe use of chemicals. In addition, all key personnel (operators, supervisors and quality technicians) must be certified by recognized bodies such as ENAC or AENOR, according to standards such as GMP+ or ISO 22000.
The performance of the hygienic culture can be measured by means of indicators (KPIs) that allow monitoring and continuous improvement.
In reference plants, it is considered optimal to maintain more than 95 % of the microbiological samples in compliance, and a compliance of more than 98 % in the scheduled cleaning and disinfection activities.
Likewise, the goal of “365 days without pollution incidents” has become a standard of excellence in the sector.
In parallel, internal audits must ensure that 100 % of personnel with critical functions are certified in food hygiene and safety.
These metrics -inspired by international guides such as the IFIF Biosecurity Guidance 2024 and the GMP+ B2 Standard- provide a quantifiable view of the organization's hygiene maturity and allow you to demonstrate, with evidence, the effectiveness of your management system to audits or international customers.
Historical cases
Historical incidents demonstrate the global impact of poor feed hygiene:
- Dioxin crisis (Belgium, 1999): contamination by industrial oils; more than 500 million € in losses and temporary suspension of exports.
- Melamine contamination (China, 2008): chemical adulteration to simulate protein; more than 300,000 people affected and multiple deaths.
- Salmonella outbreaks (EU, 2010-2020): following strict implementation of HACCP controls, outbreaks were reduced by more than 50 % (EFSA).
These crises prompted the creation of stricter international frameworks and the adoption of harmonized biosecurity programs (FAO-IFIFIF).
Economic and competitive benefits
Investment in industrial hygiene generates tangible benefits in operational efficiency and risk reduction. Case studies published by manufacturers and technology providers show reductions of 20-50% in cleaning time and relevant water and chemical savings by optimizing CIP and standardizing protocols, with increases in available capacity and sustainability improvements. Savings and return depend on the process, degree of automation and starting point, but the trend is consistent: less downtime, less consumption and better traceability.
In parallel, the mitigation of risks linked to key contaminants in the sector, such as mycotoxins (FAO/EEA confirm significant presence, (frequent detection and exceedances in relevant fractions of crops) and Salmonella (high persistence in dry environments), provides business continuity and reputational resilience, critical factors in audits and B2B relationships.
Hygiene in the feed industry is a strategic investment that combines safety, productivity and business reputation.
Complying with legislation and adopting state-of-the-art technological systems -CIP, IoT, UV-C, HEPA filters- increases competitiveness and ensures safety along the entire “farm-to-table” chain.
In a global context where traceability and biosafety requirements are increasing, factories that are committed to prevention, digitalization and hygienic culture will be better prepared to face international audits and consolidate the trust of customers and consumers.